News Archive
RESEARCH HIGHLIGHT - A novel tau fold in the neurodegenerative disease corticobasal degeneration
Corticobasal degeneration (CBD) is a neurodegenerative disease involving the cerebral cortex and basal ganglia. CBD belongs to a family of diseases called tauopathies in which the protein tau forms abnormal filaments. The LMB team has now solved the first structures of tau filaments from the cerebral cortex of patients with CBD. Importantly, they have also shown that these structures are different from those they previously solved for tau assemblies from Alzheimer’s disease, Pick’s disease, and chronic traumatic encephalopathy (CTE).
Tau protein is found in six different versions, or isoforms, that are produced by alternative mRNA splicing. These isoforms differ in the presence or absence of a sequence of amino acids near the start of the protein chain and the inclusion or exclusion of a section of the microtubule binding domain nearer the end of the protein. The microtubule binding domain is formed of three or four repeating sequences of amino acids, depending on whether this section is excluded or included. Inclusion of this repeat in three of the tau isoforms results in versions with four repeats (known as 4R), whereas exclusion in the other three isoforms leaves those with three repeats (3R).
There are more than 20 different tauopathies, with Alzheimer’s disease being the most common. Tauopathies can be categorised based on which isoforms of tau are incorporated into the filaments: 3R only, 4R only, or 3R and 4R. After Alzheimer’s disease, 4R tauopathies are the most common form of this family of diseases. In this Nature publication, the LMB team has now solved the first structures of tau filaments from a 4R tauopathy, CBD.
Every tauopathy for which a tau filament structure has been solved has a unique disease-specific fold. Structures of filaments from different tauopathies may be useful for the development of tracer compounds that are specific for the folds of tau that can be used for diagnosis in live patients. Understanding how and why tau assembles into different disease-specific structures will also be beneficial for the development of new therapies. It might, for example, be possible to design drugs that stop filaments forming in order to stop the progression of neurodegeneration.
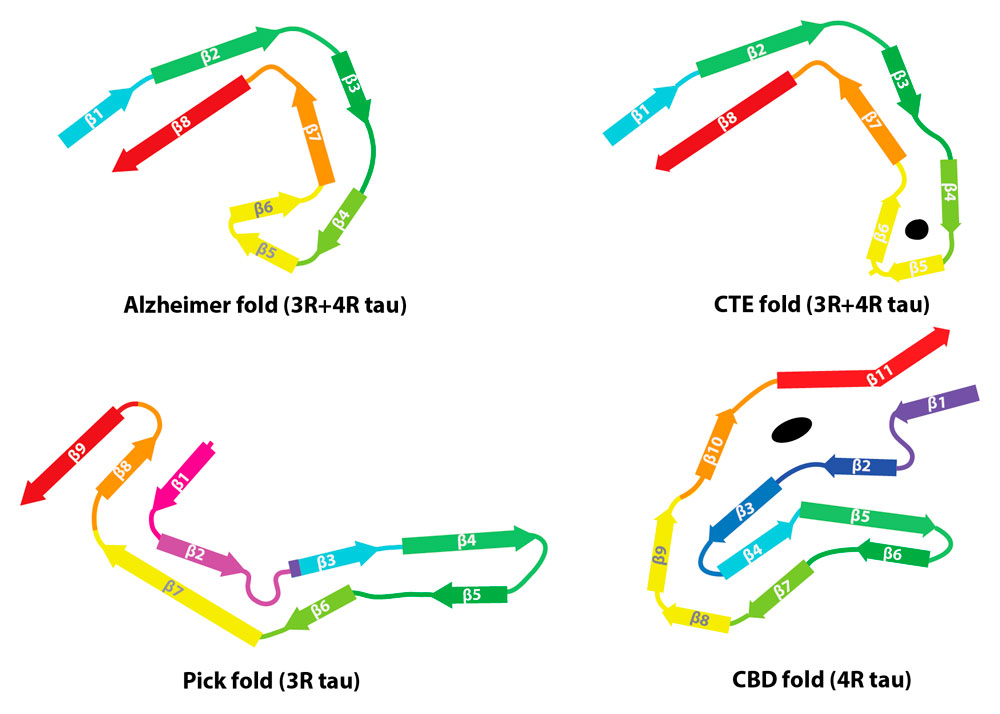
Schematic representation of the four known tau filament folds: from Alzheimer’s disease, Pick’s disease, CTE and CBD.
This project receives funding from the Innovative Medicines Initiative 2 Joint Undertaking (www.imi.europa.eu) under grant agreement No 116060. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
This work is supported by the Swiss State Secretariat for Education‚ Research and Innovation (SERI) under contract number 17.00038.
The opinions expressed and arguments employed herein do not necessarily reflect the official views of these funding bodies.



